Anti-Bacteria Antibody ELISAs as Analytical Tool in Autoimmune Disease Research
Reactive arthritis is a phenotype of spondyloarthritis (SpA) that most commonly occurs in response to infection by enteric pathogens (Salmonella, Shigella, Yersinia, and Campylobacter species) or infection of the urogenital tract by Chlamydia trachomatis (C. trachomatis). Diagnosing reactive arthritis requires the onset of arthritis three days to six weeks after symptomatic enteritis or urethritis appears, with a positive culture result for either C. trachomatis or SpA-associated enteric pathogens. However, the microorganisms that trigger reactive arthritis may only be detected for a indeterminant amount time after the initial infection (1). Therefore, other techniques like PCR-based 16S rRNA sequencing to detect bacterial RNA and ELISAs to detect bacterial components or antibodies against bacteria may aid in the diagnosis of reactive arthritis. As research increasingly indicates that the gastrointestinal microbiome plays a significant role in the pathogenesis of a variety of autoimmune diseases, like rheumatoid arthritis, colitis, and Sjögren’s syndrome, assays that can reliably detect bacteria, their immune-stimulating components, and a patient’s immune response will become vital tools for understanding the causes of these complex diseases.
A recent paper sought to evaluate the usefulness of several laboratory assay techniques in the context of SpA to better understand the relationship between disease activity and immune responses. 27 SpA patients and 26 healthy controls were subjected to bacteria culture tests and 16S rRNA sequencing analysis for stool and urine samples, as well as ELISAs to detect serum antibodies against the enteric and urogenital pathogens listed previously.
The bacteria culture tests detected Escherichia coli (E. coli) in all stool samples from both groups. However, in the SpA patient group, all samples were negative for SpA associated bacteria, with the exception of one patient sample testing positive for Salmonella enterica. 16S rRNA analysis of stool samples confirmed the positive Salmonella enterica culture test, while also detecting Clostridium perfringens in six patient and an Aermonas spp. in one patient. On the other hand, culture tests of urine samples detected E. coli and Pseudomonas aeruginosa in two SpA patients.
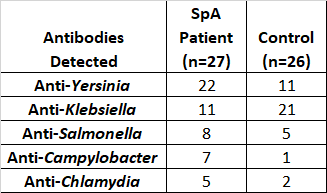
Table 1. The frequency of pathogen specific antibodies detected in the spondyloarthritis (SpA) patient group and the healthy control group.
Interestingly, the serum analysis against SpA-associated pathogens provided a much different picture. As shown in table 1, anti-bacterial antibodies against SpA-associated pathogens were detected in both the SpA patient and control group. Anti-bacterial antibodies were detected in 21 out of 26 control subjects: 14 controls with antibodies against only one enterobacteria and 19 controls with antibodies against no more than two bacterial species of interest. In contrast, anti-bacterial antibodies were detected in 22 out of 27 SpA patients: 8 patients showed antibodies against one enterobacteria and 14 patients showed antibodies against up to 4 bacteria of interest. Overall, 12 different serological antibody profiles were found in SpA patients while only 5 were identified in the control group.
As the influence the gastrointestinal microbiome comes to the forefront in autoimmune disease research, it will be important to utilize tools that can provide a complete picture of a patient’s immune response to enteric pathogens. As this study shows, serological profile analysis of bacteria specific antibodies, 16S rRNA sequence analysis, and traditional culture techniques can provide very different information on the bacterial exposure of a patient. Combining antiâ€bacterial antibody assays with other specific laboratory tests can aid both researchers and clinicians in understanding the complex mechanisms underlying autoimmune diseases. For this purpose, Chondrex Inc. provides Human Anti-Bacteria Antibody Assay Kits, Mouse Anti-Bacteria Antibody Assay Kits, and Bacterial Toxin Detection Assay Kits for elucidating the immune response of patients and experimental animals to commensal bacteria.
